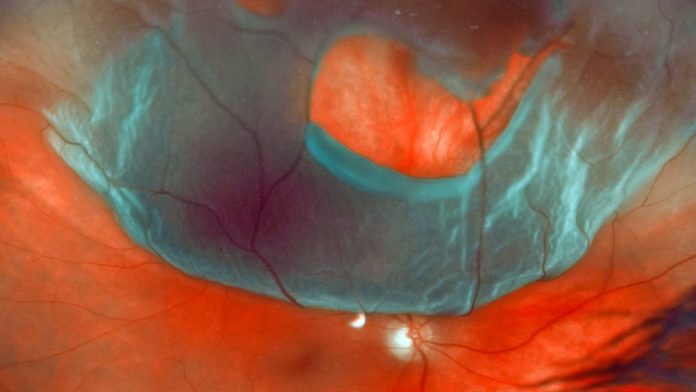
The light-sensing cells at the back of the eye form a thin layer called the retina. As we age, or in the aftermath of head trauma, this delicate sheet of cells becomes vulnerable to pressures that can pull it away from its normal position. This is called retinal detachment, and without proper treatment, the patient may permanently lose their vision in the affected eye.
Although there are established surgeries with high success rates, there are still risks, and the operation itself can significantly impact a patient’s quality of life for an extended period. There has been little innovation in this space in over a century. That’s why Toronto-based company Synakis is looking for better options to surgically re-attach the retina.
The operation involves removing the vitreous (a gel-like substance with tiny fibres that attach to the retina) so that surgeons can access the retina and repair the damage. Once the vitreous has been removed and the retina pushed back, the cavity needs to be filled with a vitreous substitute to restore functionality.
At around 85%, the success rate is good, but not perfect; either way, your life is going to be pretty uneventful for the foreseeable future.
“When your vision works properly, it’s taken for granted; when it fails, you realize just how much your quality of life is affected… it’s an unpleasant experience,” said Synakis President Alexander Baker, who completed his PhD at the Shoichet Lab in 2019.
For over a century physicians have mainly used gas — “literally filling the eye like a balloon,” said Baker. As part of the patient’s recovery, the gas bubble must be absorbed by the eye, and that can take anywhere from two to six weeks.
To keep the bubble in the right spot, patients must work with gravity by holding their head in a certain position during the recovery period (known as “posturing”). Strenuous activities like working, driving, or intense exercise are out of the question until recovery is complete.
Silicone oil is another option. These substitutes have different physical properties like density and stability which determine their longevity, and they are eventually removed because they aren’t absorbable and the oil may emulsify if it is disturbed too much or left in too long, resulting in blurry vision. Moreover, the double surgery is a further burden on the patient.
Synakis was founded with the mission of creating a stable vitreous substitute that reliably delivers clear vision, and doesn’t require weeks of inactivity after the operation. Development is underway, and the group are working towards scaling up manufacturing with the goal of getting their novel material into clinical trials.
The project began with ophthalmologist Rob Devenyi from Toronto Western Hospital, who noticed a clear demand from both surgeons and patients for a better solution, and a strong market for it. In North America, there are ~250,000 surgeries requiring the removal of the vitreous performed annually, including ocular gene therapy, and correcting macular holes and retinal detachment.
To develop a solution, Devenyi partnered with internationally recognized biomaterials scientist Molly Shoichet. Shoichet is a Research2Reality co-founder and professor of chemical engineering at the University of Toronto. She is also the co-founder of AmacaThera, a company that is developing a safe alternative to prescription opioids for managing post-operative pain.
A stable, biodegradable, and injectable substitute
After consulting with patients and surgeons in both the Toronto area and internationally, the objective became clear for Synakis: to develop a stable, biodegradable, and injectable vitreous substitute that delivers clear vision. No double surgery, no posturing, no extended recovery times, and more consistent outcomes.
Following years in the lab spent optimizing the hydrogel formulation, the team progressed to testing the material in animal models.
“We knew we had to test this in vivo as you want to make sure the risk is as low as possible. We’ve done lots of animal testing in rabbits to show there is no concern about the material degrading, and that it’s tolerated, i.e., the eyes are still healthy after,” said Baker.
“With the results of our research recently published in Biomaterials — a peer-reviewed journal — this builds our reputation as leaders in developing better vitreous substitutes.”
The team are now focused on accumulating enough funding so they can demonstrate the feasibility of scaling up manufacturing efficiently — a mandatory part of the regulatory process needed to gain approval for clinical trials.
The experience of developing a company from the ground up was a formative one for Baker, who has been central to the project’s development from both a scientific and business perspective.
“Shifting to the role of president with Synakis made me learn more about business development,” commented Baker. “Molly is a fantastic mentor because she has plenty of experience on boards, but also in the commercialization space.
“Before leading this project, I didn’t have a lot of experience working with clinicians, and it takes a multi-disciplinary team to solve these big challenges. At Synakis, we’ve been able to establish that team.”
To learn more about Synakis, readers are invited to reach out to Alexander Baker by email.