Prostate cancer is the most common type of cancer among Canadian men, and according to the Canadian Cancer Society, one in nine will be diagnosed with prostate cancer in their lifetime and one in 29 will die from the condition. Effective treatments exist, but they come with a major cost: infertility, incontinence, erectile dysfunction, and reduced sexual desire are all potential side effects.
Now, a Canadian innovation could deliver the best of both worlds. TULSA-PRO is a medical device that precisely targets cancer cell regions of the prostate while sparing healthy tissue. In doing so, the device either limits or prevents the negative side effects associated with traditional treatments like radiation therapy and prostatectomies (partial or full removal of the prostate).
The treatment is fast, requires no incisions, and is minimally invasive, making things a lot easier on the patient both during and after surgery. The company behind the technology, Mississauga’s Profound Medical, may offer patients hope that they can overcome the disease without suffering these complications.
“Profound’s exciting non-invasive therapy technology has the potential to bring significant benefit to a large number of prostate cancer patients,” said Baldev Ahluwalia from GE Healthcare, a partner group assisting with distributing the technology worldwide.
Killing cancer tissue in only a few hours
Ablation is an established practice for targeted treatment of certain solid cancers of the liver, lungs, and kidneys by using a small and focused probe. Some use lasers, and others are armed with thermal technology, in effect heating or freezing cancer cells to death. Profound’s technology aims to expand this type of treatment to prostate cancer to spare more of the healthy tissues around the prostate.
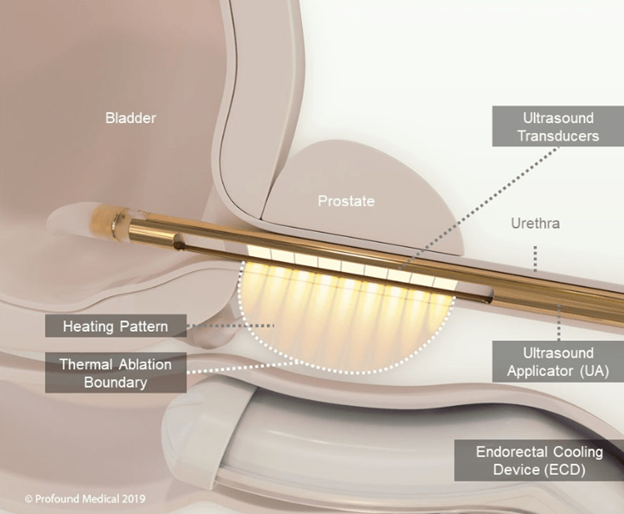
TULSA-PRO can be inserted in a minimally invasive procedure through the urethra, meaning no incisions are needed. Once in place next to the prostate, the probe uses directional ultrasound to produce heat in a controlled pattern across the wall of the urethra, thereby ablating the target areas. It simultaneously protects the patient’s urethra and rectum with a cooling device, preserving healthy function and dramatically reducing the risk of side effects commonly associated with prostate cancer treatment.
Clinicians can make use of the real-time MRI feedback to observe the process and customize each patient’s treatment plan, and there’s also ongoing temperature feedback to ensure a gentle and precise ablation process.
Treatment with TULSA-PRO is fast, taking as little as a few hours to complete. This makes it a same-day service, so it’s “practically immune” from the restrictions imposed by the pandemic that are forcing hospitals to delay conventional treatments due to long recovery times.
Expansion into the US and worldwide
In the US, Profound received clearance from the FDA in 2019 following the success of the TACT pivotal clinical trial, which demonstrated that TULSA-PRO provides safe and effective prostate tissue ablation with minimal adverse effects and low rates of residual disease.
Profound is currently using their funding to expand the commercialization of their technology in the US and further afield. RadNet, a leading provider of outpatient diagnostic imaging services in the US, signed with Profound to install three TULSA-PRO systems at its imaging centres in Los Angeles in January 2020. A worldwide license was signed in December 2020 with GE Healthcare, a global medical technology and digital solutions innovator, which will allow Profound to expand by making use of GE’s sizeable stock of MRI scanners installed worldwide.
Last year was unkind to businesses generally, but Profound continued to make serious headway with revenue totals clocking in around $9.7 million, marking a 32% year-over-year growth figure.
“We believe the strong sales performance in the fourth quarter, which was achieved in the face of continuing COVID-19 headwinds for the medical device industry, speaks to the strength of both our technology and business model,” commented Arun Menawat, Profound’s CEO to Globe Newswire.
“While we remain cautious about the scope and pace of U.S. TULSA-PRO commercial adoption in the near-term due to the pandemic, we are also energized going into 2021.”