I remember making paper snowflakes back in elementary school: folding up the white sheet, cutting out tiny, intricate shapes, and finally the moment of truth when the paper was unfolded and a beautiful snowflake emerged.
But for Ken Libbrecht, Chairman of the physics department at Caltech, making snowflakes is not a thing of the past. Only now, instead of using paper, Libbrecht builds real custom snowflakes using precisely controlled temperature and humidity conditions.
And yes, some of them are identical.
Whipping water vapour into shape
Snowflakes, or more precisely, snow crystals, are not actually formed by freezing water. Rather, they form under conditions where water vapour deposits on a small particle, like a piece of dust, and goes directly from the gas to the solid state.
The precise shape the vapour takes on is governed by the weather conditions and there’s a sweet spot around -15°C where the large, tree-like, picture-perfect snowflakes form. The rest of the time we get things like hexagonal plates, columns or, needles.
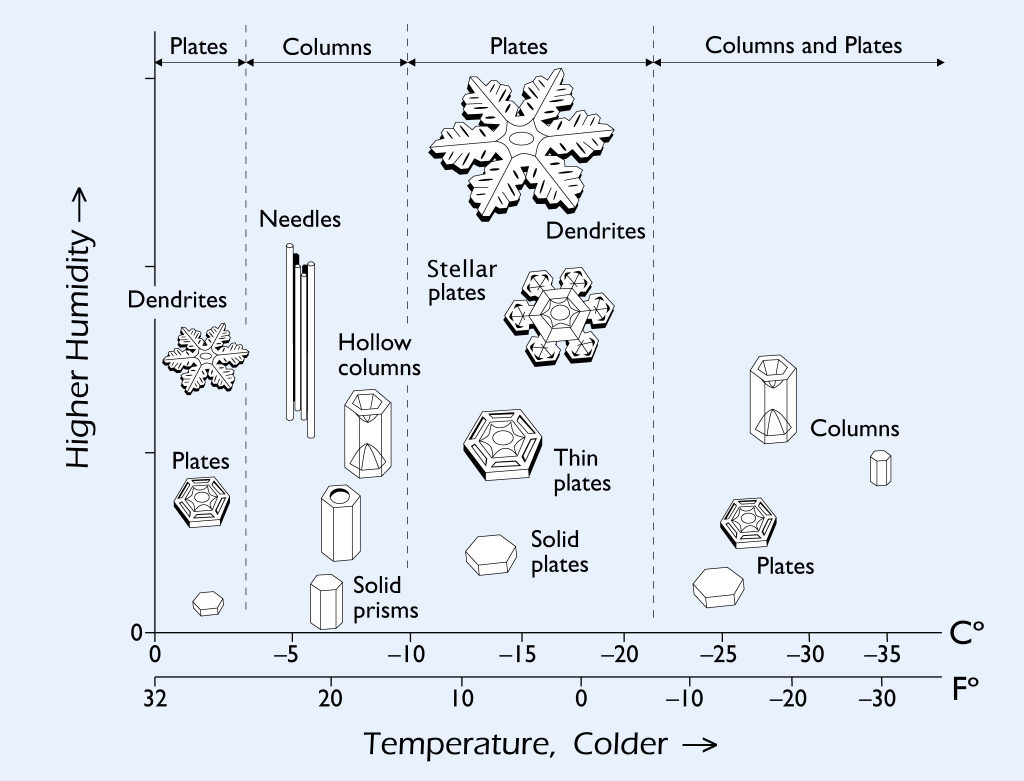
But there’s some things that don’t change, like the six-fold symmetry of a single snow crystal. That’s because the V-shaped water molecules in ice arrange themselves into an ordered, hexagonal crystalline lattice. The snow crystal grows from each of its six corners, resulting in a six-sided or six-armed snowflake. The exact way the crystal grows is, again, governed by the exact temperature and humidity conditions. As the nascent flake is buffeted through different areas of the cloud, it experiences a different set of conditions, resulting in a slightly different pattern of vapour deposition and a different shape. Because no two snowflakes take the exact same path to the ground, all of them are unique.
Unless, of course, you’re a physicist with a custom-made snowflake machine.
Designer snowflakes
Libbrecht starts each design with a tiny hexagonal ice crystal. Then, using over $10,000 worth of hardware designed specifically for this purpose, he deposits the ice crystal on a sapphire pedestal and grows it by gently wafting in perfectly controlled, cold, moist air. Depending on the shape, a single crystal can take up to 3 hours to grow. The reward? Crisp, clean edges and corners that you would never see on a natural snowflake.
His latest project is growing “identical-twin” snowflakes that are nearly indistinguishable to the human eye – just like human identical twins.
“Identical twin” snowflakes being grown in Ken Libbrecht’s lab. This is not a computer simulation, nor it is PhotoShop trickery. Movie is about 60x sped up. Video courtesy to Ken Libbrecht at snowcrystals.com
But snow is not the only “cool” winter phenomenon you can study in the lab.
Professor Stephen Morris from the Department of Physics at the University of Toronto has his own specially designed, “Rube-Goldberg-like machine” to grow icicles.
Patterns emerging
Although, at first glance, icicles are not as complex as snowflakes, they have their own intricacies. For instance, have you ever seen an icicle with ripples?
Icicle ripples are part of a larger field of study in physics called emergent phenomena – the appearance of regular, ordered structures under conditions where you wouldn’t normally think they would appear.
Professor Morris describes emergent phenomena using the example of sand ripples on a beach.
“You wouldn’t think anything organized would come out of that – sand bounces around very randomly and the wind is turbulent – but in fact these beautiful little ripples appear. And the explanation cannot be found by examining an individual grain of sand very carefully, because it lies at some level of organization which is much bigger.”

Similarly, looking at each water molecule individually, you would never predict ripples on an icicle. Yet, there they are.
So Morris and his team built a machine to precisely control icicle growth and study how the icicle size, shape, and morphology is governed. The machine is connected to a camera, so they can watch the icicles form in real time.
In initial experiments, the Morris lab used regular tap water, and out emerged beautifully rippled rods. But Toronto tap water has all kinds of things in it, so in an attempt to be rigorous, more icicles were made using distilled water.
The resulting icicles were smooth.
“[We] went looking for a phenomenon and when [we] made a precise experiment the phenomenon disappeared!” laughs Morris.
It turns out it takes just a tiny amount of salt, about one one-thousandth of a percent (0.001%), to make ripples on an icicle, and it all has to do with the resulting tiny change in the water’s freezing point – just like putting salt on the road lowers the melting temperature of ice and snow.
Since those first experiments, the Morris lab has grown hundreds of icicles to try and elucidate the equations governing their formation. And he’s documented them all in a publicly available icicle atlas.
11 random icicles from the Icicle Atlas. Movie courtesy of Stephen Morris.
A world of icy treasures
In his icicle atlas, Morris compiles photographs of each icicle along with a description of the conditions under which it was formed. But even if you’re not interested in the physics behind these phenomena, the time lapse videos of icicles forming are mesmerizing. For some icicles, Morris even provides 3D printable files so you can choose your favourite icicle and create a 3D replica.
Just in case you didn’t have enough Canadian winter.









































