Exercise has a powerful impact on bone health — any kind of movement places pressure on our bones through the complex interaction of forces like gravity and our muscle contractions. Regular interaction with these forces creates more resilient bones, but low activity does the opposite, and this is an issue for people with limited mobility (including astronauts).
Researchers from Shriners Hospitals for Children – Canada and McGill University have unearthed a key insight into a complex bodily process: how our bones self-repair following mechanical stress. Their work sheds light on how pressures from exercise are dealt with by the body to maintain bone health.
When bone cells start breaking down from mechanical stress, a series of molecular events is triggered. Calcium is a key player in this process, as is adenosine triphosphate (ATP) — a primary molecule for chemical energy storage used to carry out all kinds of biochemical processes that is sometimes called the “energy currency of life”. Their release is one of the first cellular responses in the process of repair and adaptation.
However, it was unknown how mechanical forces led to ATP release, which inspired these researchers to dig deeper.
“The goal of our study was to examine the mechanism of ATP release from mechanically stimulated bone cells,” says lead author Nicholas Mikolajewicz of McGill University and Canada Shriners Hospital in a press release.
“We realized early on that the membrane of bone cells needs to be disrupted to increase calcium levels, and soon after we became interested in how these injuries affect ATP release.”
Regular micro-injuries train bone cells to self-repair faster
The team first explored the timing of ATP release and found that it appeared mere seconds after these injuries, with calcium increases in nearby cells following.
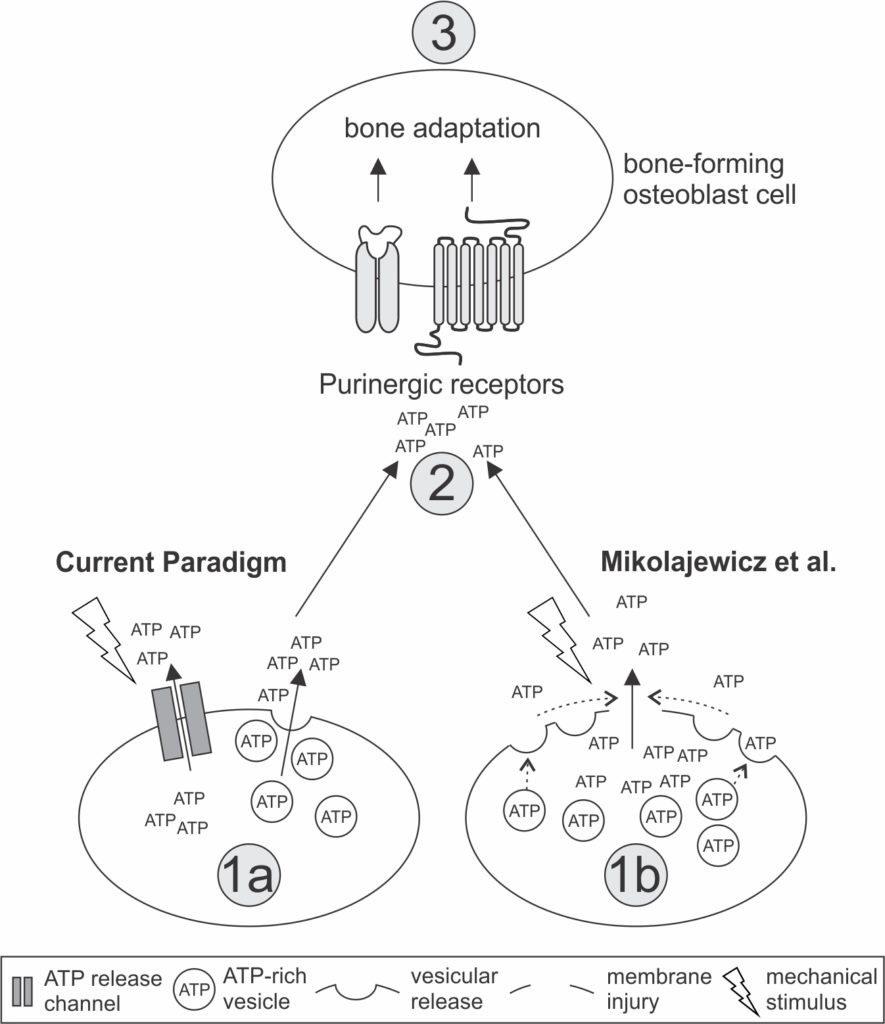
Next, they investigated whether ATP exits the cells through vesicles — small membrane sacs in the cell which are released during stimulation — following regular levels of mechanical stress.
Lab-grown bone cells were stimulated using either a glass needle or placed in a turbulent fluid that simulates forces experienced by the body. A selection of dyes was used to track the release of these vesicles.
120 second time-lapse of signal transmission between bone cells following the application of mechanical force to a single bone cell. Courtesy of McGill/Shriner’s Hospitals for Children Canada.
To their surprise, the researchers found the opposite of what they had theorized: the more vesicles that were released, the less ATP was observed.
“The previous theory wasn’t consistent with what we were observing, and that’s what turned us onto micro-injuries,” adds Mikolajewicz.
While ATP is present in vesicles, the primary release of ATP flows through membrane openings caused by micro-injuries, and it goes on to trigger further molecular events.
The presence of a calcium build-up following membrane injury ‘switches on’ the molecule protein kinase C (PKC), which in turn controls vesicle release. The role of vesicles is to ‘seal the hole’ in the membrane by fusing with the membrane wall, preserving stores of ATP.
“Our first research project shows that bone cells adapt to physical forces, such as encountered during exercise, and that the more the bone cells suffer from micro-injuries, the more quickly they repair themselves,” concludes senior author, Svetlana Komarova, PhD, from Shriners Hospitals for Children – Canada’s Research Centre and Associate Professor at McGill University.
Bone repair disorders need personalized treatment
For the second half of their research, the team conducted a meta-analysis of over 250 studies involving mechanically-stimulated ATP release.
Multiple important discoveries were made in this section of the research. For the first time, the volume of ATP release from each healthy cell was quantified: around 24 million molecules. They also discovered that the type of force applied determines the route the ATP takes as part of its release.
Notably, however, they found that different classes of diseases have a significant effect on ATP release.
“There’s so much literature out there but no one’s ever tried to synthesize all the data,” says Mikolajewicz. “We found that a lot of conditions with patterns of inflammation and injury had higher ATP release, while metabolic and hereditary diseases issued lower amounts.”
An impressive step forward knowledge-wise, but what about the practical aims of this research?
“The hope is that we will be able to pinpoint the events that need to happen for bones to respond and adapt,” says Professor Komarova.
“This way, we can create personalized treatments for people whose bone health is at risk such as many of the patients we see at Canada Shriners Hospital; paralyzed people, the elderly, and other vulnerable individuals as well as astronauts.”