Canadian researchers at Polytechnique Montréal, Université de Montréal, and McGill University have succeeded in reprogramming magnetic bacteria to carry nearly 30 times more anti-cancer drug to tumours than standard chemotherapy (up from 2% to 55%) in a mouse model of cancer. The results were published this week in Nature Nanotechnology.
While it remains to be seen whether this results in more effective treatment, or whether these results can also be seen in human patients, these promising nanorobots may be better at knocking out cancer, while reducing toxic side effects.
Why nanorobotics?
The idea to use magnetic fields to direct drugs to tumour sites is not new, but to navigate through the small capillaries that carry blood to the tumour, drug loaded particles have to be tiny – as small as 2 microns – and at that size, the technology isn’t available create a magnetic field strong enough for guidance.
To overcome this hurdle, Sylvain Martel, professor of biomedical engineering at Polytechnique Montréal, looked to nature for a self-propelled option.
Meet Magnetococcus marinus
In nature, bacteria called Magnetococcus marinus lives in the deep waters of an estuary in Rhode Island. Here, they follow magnetic fields to get to the low-oxygen areas where they prefer to live, swimming there using whip-like flagella. They have a natural chain of magnetic iron oxide nanocrystals that act like a compass needle for guidance.
This natural set of features made Magnetococcus marinus an ideal candidate for a cancer-seeking nanorobot: they can naturally follow magnetic fields and could be directed using computer-controlled magnetic fields, and once near a tumour, they self-navigate to the low oxygen environments that characterize the most active areas of tumour growth. They are also fast swimmers, moving about ten times the speed of average bacteria, swimming the length of their own bodies 200 times each second.
Drug-carrying bacteria deliver payload, then self-destruct
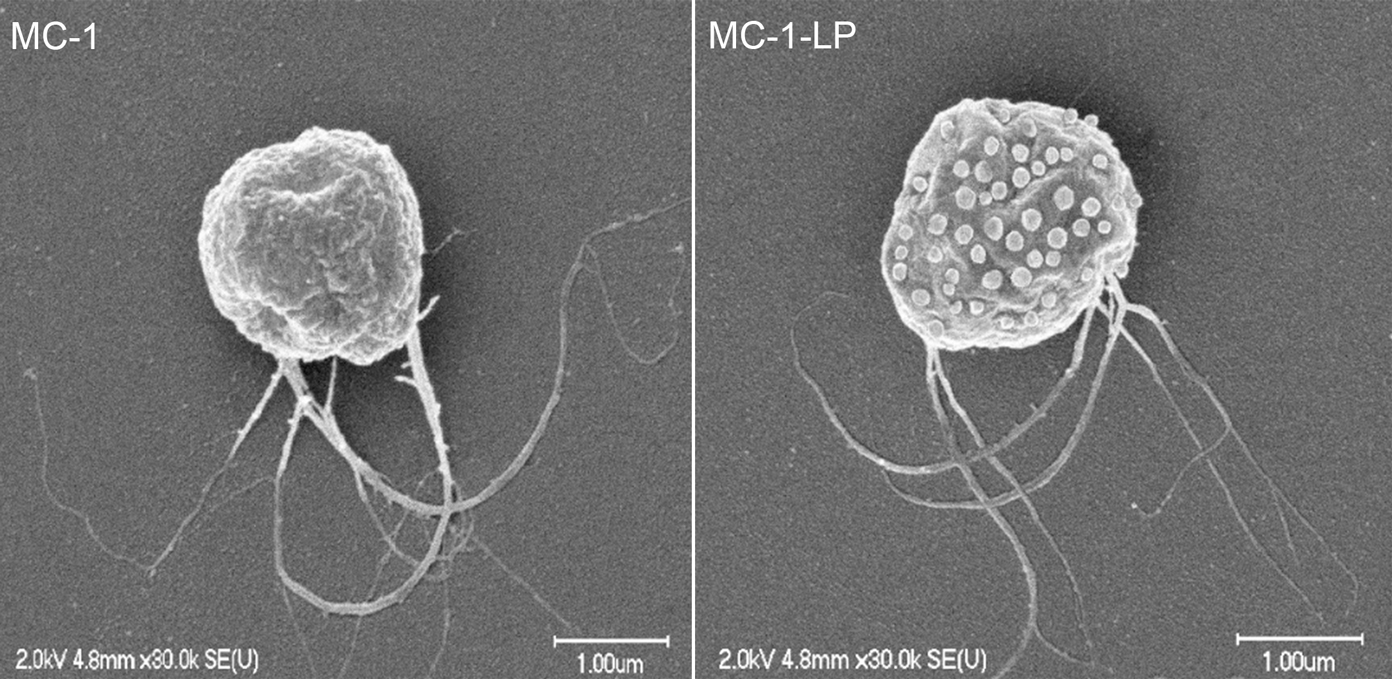
Martel and his collaborators attached liposomes, sacs filled with an anti-cancer drug, to a particular strain of Magnetococcus marinus called MC-1. They carry the drug-filled liposomes on their backs as they swim through the bloodstreams of mice with colorectal cancer.
Magnetococcus marinus MC-1 as found in nature (left) and modified to carry drug-loaded liposomes on its surface (right)
Credit: The NanoRobotics Laboratory, Polytechnique Montreal
After 30 minutes, an average of 55% of the injected bacteria made it to the tumour site, a huge improvement over the 2% achieved by standard chemotherapy. By this point, unaccustomed to high temperature of mammalian body heat, the bacteria die and release their payload.
So far, increased targeted drug delivery has been shown in mice, but before moving to the clinic, these nanorobots will need to prove that this allows them to attack tumours more effectively. The results will also need to be demonstrated in human patients.